Lyme Disease
Lyme disease refers to the clinical signs and symptoms related to infection with the spiral bacteria Borrelia burgdorferi, or Lyme borreliosis. It is the most common tick-transmitted infection in the United States and Europe, with more than 30,000 new cases reported to the CDC per year. Results of two studies suggest the actual number of diagnoses cases of Lyme disease in the US is around 300,000.1 This discussion will focus on the assessment and diagnosis of Lyme borreliosis contracted in the United States.For most patients, the recognition and diagnosis is fairly straightforward. However, the diagnosis can be challenging for several reasons. Symptoms of infection may be non-specific, and overlap with other inflammatory medical conditions. Diagnosis most commonly relies on demonstrating a specific host immune response by serology, but specific PCR detection of B. burgdorferi is commonly used for complicated patients.
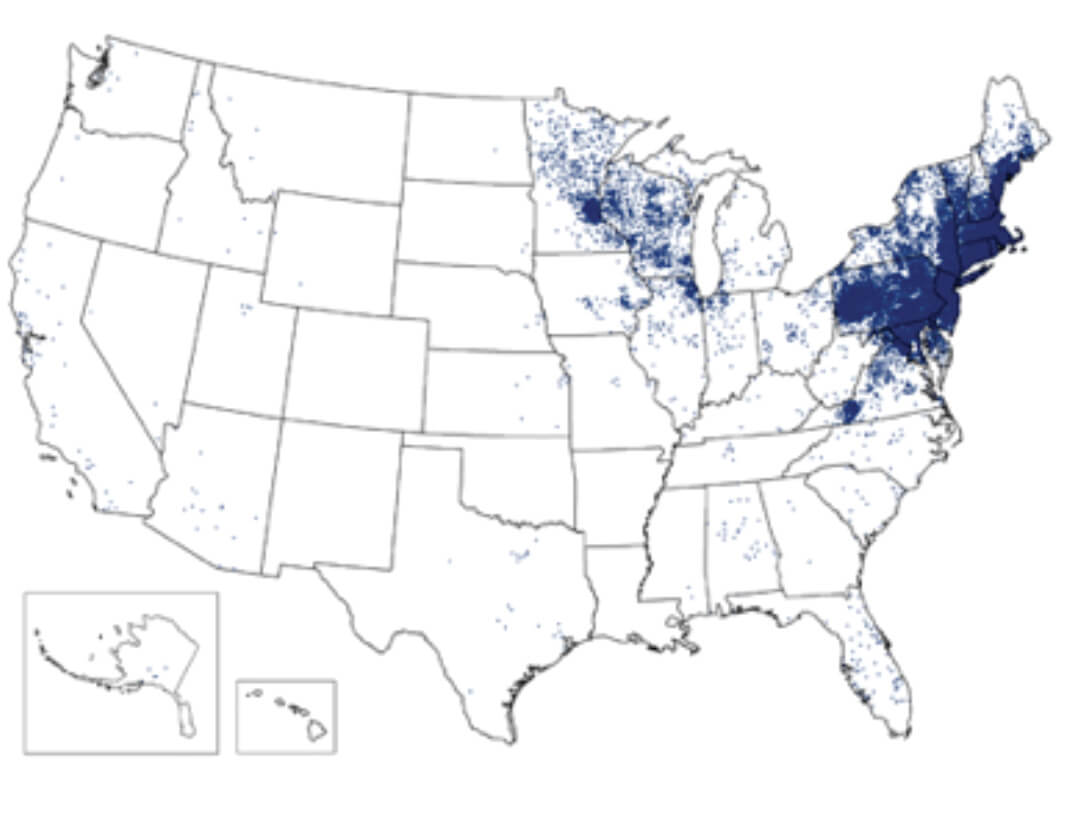
Figure 1. 1 dot placed randomly within county of residence for each confirmed case.Ixodes scapularis ticks have three stages (Fig. 2). All stages are critical to maintaining the transmission cycle. Most human infections are transmitted by nymph phase ticks, which feed aggressively in the spring and summer months. Because of their small size (<2mm), nymphal ticks are more difficult to detect after attachment, and are, therefore, more likely to remain attached for the 36 to 48 hours needed for effective transmission of infection.
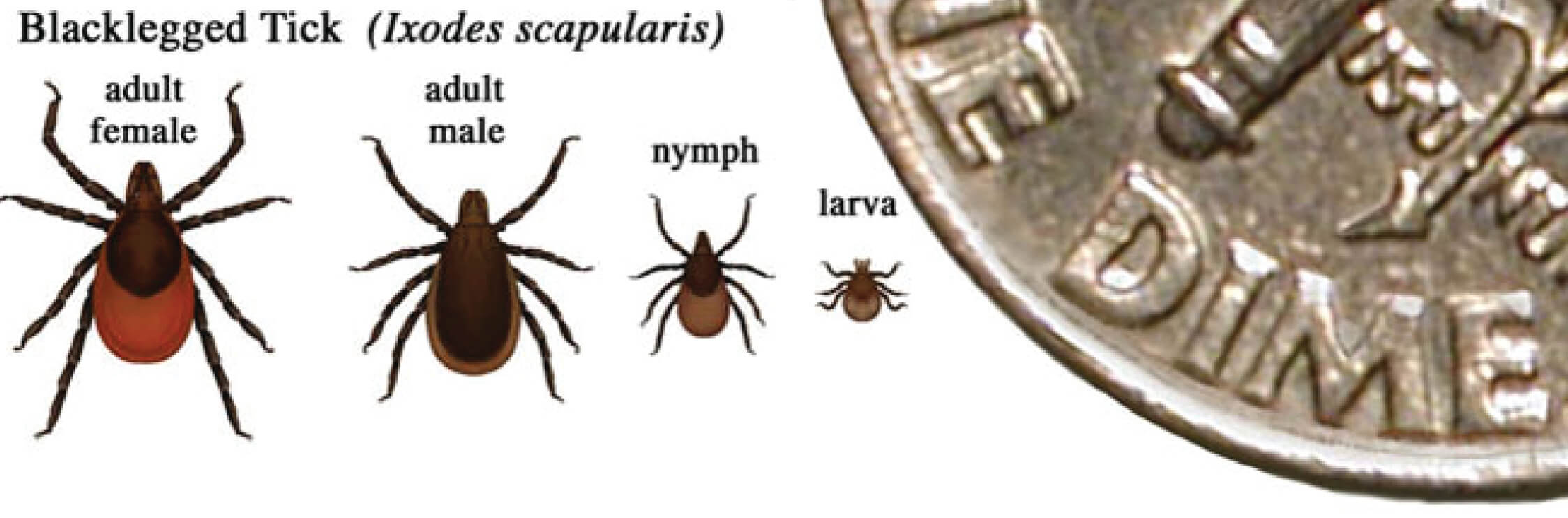
Figure 2. In general, adult ticks are approximately the size of a sesame seed and nympphal ticks are approximately the size of a poppy seed.
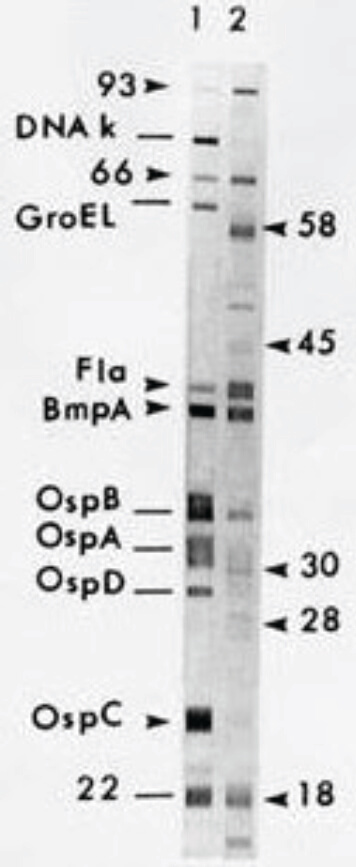
Figure 3. Positive Western blot.
Life cycle
Borrelia burgdorferi has a complex life cycle that requires sequential infection of an arthropod vector, ixodid ticks, and a variety of warm-blooded, usually mammalian, hosts. Humans may be infected as a dead-end host through the bite of an infected tick. Approximately 95% of cases reported in the U.S. occur in heavily wooded areas of the coastal states of the Eastern United States, especially New England, as well as Minnesota and Wisconsin (Fig. 1). This distribution reflects the habitat of the normal host species for B. burgdorferi: most commonly Ixodes scapularis ticks, and a variety of small and large woodland mammals.Reported Cases of Lyme Disease, United States, 20141

Figure 1. 1 dot placed randomly within county of residence for each confirmed case.Ixodes scapularis ticks have three stages (Fig. 2). All stages are critical to maintaining the transmission cycle. Most human infections are transmitted by nymph phase ticks, which feed aggressively in the spring and summer months. Because of their small size (<2mm), nymphal ticks are more difficult to detect after attachment, and are, therefore, more likely to remain attached for the 36 to 48 hours needed for effective transmission of infection.
Relative sizes of blacklegged ticks at different life stages2

Figure 2. In general, adult ticks are approximately the size of a sesame seed and nympphal ticks are approximately the size of a poppy seed.
Organism and pathophysiology
A spirochete, Borrelia burgdorferi is significantly different than common bacterial pathogens. Organisms have a corkscrew morphology, 20–30 μ in length, but only 0.2–0.3 μ in diameter. Their narrow diameter prevents them from being detectable by routine microscopic techniques.B. burgdorferi has a small genome and lacks critical biosynthetic genes; these organisms are obligate parasites unable to replicate independently in the environment. There are no genes encoding toxins or degradative enzymes that cause direct tissue damage in infected hosts. On the other hand, there are a remarkable number of genes encoding surface-exposed lipoproteins. Up-and-down regulation of the expression of these genes allow the organisms to adapt to the varied sites of infection in their arthropod and vertebrate hosts, and to evade the immune responses encountered in their warm-blooded hosts. There is regional and local antigenic variability among strains of B. burgdorferi, and prior infection by one strain may not provide immunity to infection by other strains.Clinical signs and symptoms
Early localized disease is characterized by rapid local proliferation of B. burgdorferi at the site of the tick bite. The clinical manifestation is the development of the characteristic skin rash: erythema migrans (EM). EM occurs in ~80% of patients, but many may not recall a tick bite. This localized skin rash appears within 30 days after the bite, usually within 7–14 days. There may be induration at the site of the bite, followed by slowly expanding, often asymmetric, erythema around the central lesion. The rash is not painful, but may burn or itch. Primary lesions may reach diameters of 10 inches, and clearing inside the advancing edge, the bull’s-eye sign develops in many, but not all patients. Lesions most commonly occur in or near the axilla, popliteal fossa, groin, or belt line. Other manifestations are usually mild. The ESR may be elevated, but other laboratory abnormalities are less common, and may indicate need to consider other causes, like anaplasmosis.Early disseminated disease is usually manifested weeks to months after primary infection, and some patients do not recall a primary, localized infection. Early disseminated disease often presents with cutaneous, neurologic, and/or cardiac manifestations.- Cutaneous Patients, especially children, may present with multiple secondary EM lesions. These annular lesions are usually smaller and symmetrical, and do not imply other sites of tick bites.
- Neurological Aseptic meningitis, cranial neuropathy (especially facial palsy), and radiculopathy (sensory or motor) are common findings, in combination or alone.
- Cardiac Atrioventricular heart block is most common, but myocarditis or pericarditis, usually mild, may be seen.
- Other Ocular manifestations may include conjunctivitis, or more rarely iritis, keratitis, or other abnormalities. Patients may experience significant systemic symptoms, like malaise and fatigue, during early disseminated disease.
- Late Lyme arthropathy most commonly presents as intermittent or persistent large-joint inflammation, most commonly involving the knee. The associated tendons or bursae may also be affected.
- Late neuropathy most commonly manifests as “Lyme encephalopathy”, characterized by mild cognitive disturbance, or polyneuropathy, characterized by radicular pain or distal paresthesias.
Other manifestations
Nonspecific signs and symptoms are often reported and may occur at any phase of infection. These include: fatigue, myalgias and arthralgias, headache, or neck stiffness. Anorexia, regional lymphadenopathy, and fever are less commonly reported.Post-Lyme or chronic Lyme syndrome is used to describe symptoms that persist for months after appropriate antibiotic therapy. After treatment, typical patients show a gradual resolution of symptoms, and falling antibody titers. Certainly, disease symptoms may persist because of ineffective antibiotic treatment, or patient non-compliance. But even after effective therapy, some symptoms, especially those associated with severe disease, may resolve only slowly because of continued inflammation of damaged tissue. The question of persistent or antibiotic-resistant infection has been raised, but carefully performed studies have failed to demonstrate organisms in affected tissues. Furthermore, case-controlled, double-blind studies of additional or intensified courses of antibiotic treatment have not shown clinical benefit for patients with persistent symptoms, compared to untreated controls.8,9 Other diagnoses should be considered for patients whose symptoms persist for longer than 6 to 12 months after completion of treatment for Lyme borreliosis.Diagnosis3,7,10The decision to perform laboratory testing aimed at the diagnosis of Lyme borreliosis should be based on several factors:- Symptoms - Does the patient have signs and symptoms consistent with Lyme borreliosis?
- Geography - Has the patient been in a region where Lyme disease transmission occurs?
- Behavior - Does the patient have activities that are associated with exposure to ticks? Note that the absence of a tick bite does not exclude a diagnosis, as many Lyme patients with exposure risk do not remember a bite.
- A positive WB confirms B. burgdorferi infection.
- A negative WB supersedes positive or equivocal results by screening serology, and the evaluation for B. burgdorferi infection is considered negative.
Positive Western blot4

Figure 3. Positive Western blot.
Serology notes
- It is critical to perform both steps of the two-step process. For example, performing WB without initial screening may be associated with a false positive rate greater than 5%.
- IgM antibodies are usually detectable within two weeks of primary infection, and IgG antibodies are usually detectable within six weeks of infection. Testing during early, localized disease may be associated with a “false” negative result, and retesting should be considered for patients with a high suspicion of Lyme borreliosis.
- Patients with EM who are treated empirically during the early phase of infection may not seroconvert, but many do demonstrate seroconversion. This seroconversion does not imply a failure of therapy in compliant patients with resolution of symptoms.
- Specific serological response can be demonstrated in virtually all patients with late or chronic manifestations of Lyme borreliosis.
- Positive routine serological testing may not be able to definitively distinguish between current, active, and past infections. IgM antibodies may remain positive for years after effective therapy, and cannot be relied on as a sign of acute infection. Interpretation of results must be informed by other clinical and laboratory data.
- There may be some interlaboratory variability in results of Lyme serology testing, but this occurs mainly in specimens collected early in infection. Interlaboratory IgG results agree for virtually all patients with disease greater than 3 months when tested using CDC criteria and standardized testing methods.5
- Borreliosis similar to Lyme occurs in Europe and Asia. Some cases are caused by B. burgdorferi, others by related Borrelia species. Special testing may be needed to document borreliosis contracted outside the USA.
Other diagnostic tests
- Culture methods, though described, have limited utility and are not widely available for diagnosis.
- CSF PCR may be positive in patients with acute aseptic meningitis in early, disseminated disease.
- Synovial Fluid PCR may be positive in patients with Lyme arthritis prior to antibiotic therapy, and may be helpful in confirming a diagnosis in complicated patients.
- Alternative diagnostic test results must be interpreted in the context of routine serological testing results, as well as the clinical presentation of the patient. False positive and false negative may occur, and results must be interpreted carefully.
- Criteria for immunoblot interpretation developed “in-house.”
- Urine B. burgdorferi antigen testing, including “reverse” immunoblot confirmation.
- Various techniques for detection of cell wall-deficient forms of B. burgdorferi, including culture, immunofluorescent staining, or cell sorting.
- Lymphocyte transformation tests, or CD57 lymphocyte quantification assays.
- Tests for detection of antibodies in synovial fluid.
Buy your own lab tests
Shop online for a Lyme Disease Test - no doctor visit required for purchase





