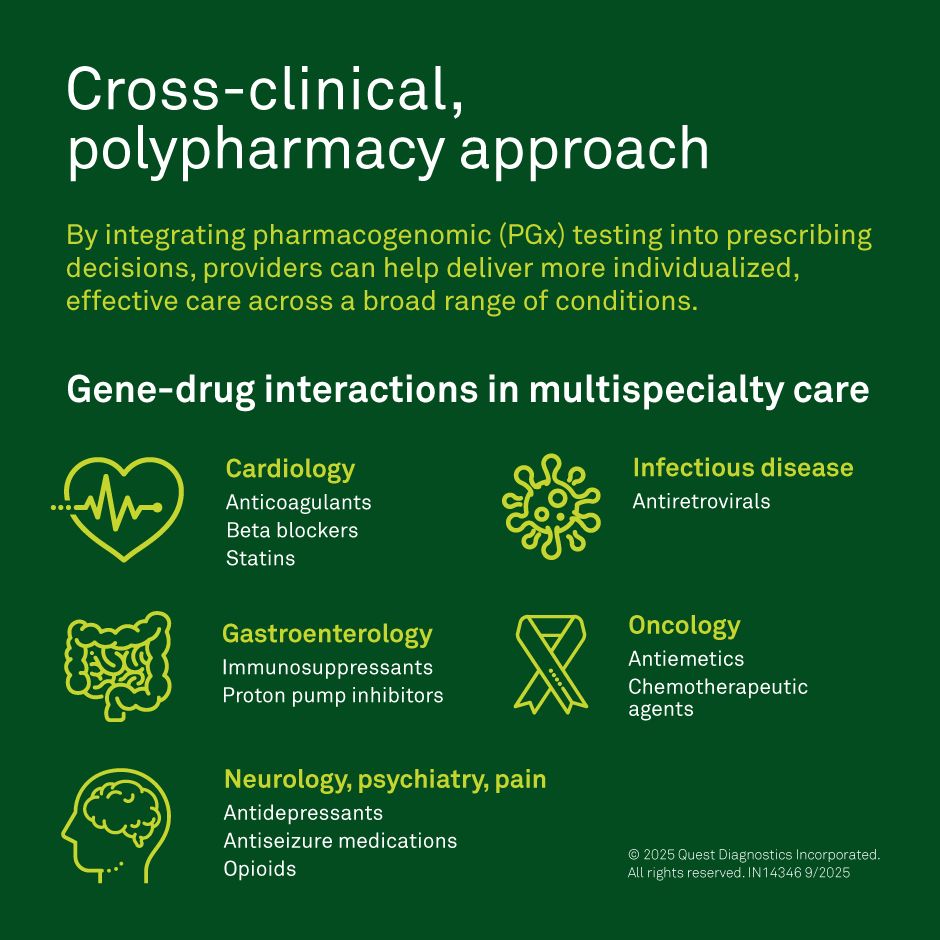
Genetic impact on prescribing strategies
Genetic variations can impact drug efficacy and response leading to added expenses for health systems
Variants in specific genes can significantly alter how a drug is absorbed, activated, or broken down—leading to reduced efficacy or increased risk of adverse effects.
Pharmacogenomics offers an opportunity for safer and more efficient pharmacotherapy—minimizing trial-and-error prescribing while improving outcomes and reducing costly hospital admissions.4